Here is an article that shows with laboratory work (scroll down) that carbon dioxide lowers the temperature of the atmosphere. Carbon dioxide thus acts as a leveling factor for the climate if increased solar radiation would make the climate warmer. Carbon dioxide counteracts global warming.
The world’s oceans are constantly in equilibrium with the atmosphere when it comes to a variety of gases. One of these gases is carbon dioxide. The world’s oceans have dissolved and store enormous amounts of carbon dioxide, about 20 million times more carbon dioxide than an annual emission to the atmosphere, all sources included (of which humans only account for 3.4%).
As the sun warms the earth more over certain periods, depending on the different sun cycles, the world’s oceans gas off carbon dioxide to the atmosphere, since carbon dioxide dissolves poorer in warmer water. With reduced solar radiation, more carbon dioxide will dissolve in the oceans, since carbon dioxide dissolves better in colder waters.
If the carbon dioxide concentration in the atmosphere increases, less water vapor (water) will be able to stay in the atmosphere and falls down as rain. Water has a high specific heat capacity (4.19 KJ / kg × K) and carbon dioxide has a lower specific heat capacity (0.84 KJ / kg × K). Good heat-accumulating water is replaced by poorer heat-accumulating carbon dioxide. The gas pressure in the atmosphere is the same. Carbon dioxide thus acts as a buffer against increased global warming.
Changed composition of the atmosphere over geological time periods?
Our atmosphere has slowly changed since the beginning of time. From the beginning it consisted of water vapor, sulfuric acid, carbon dioxide, ammonia and other unpleasant gases. The earth was initially completely uninhabitable. As geological activity eventually declined, oxygen began to be produced by the world’s oceans. Blue-green algae used the carbon dioxide dissolved in the water and created oxygen instead. Oxygen began to be stored in the oceans and slowly the gas also ended up in the atmosphere. An equilibrium was established which today means that we have an atmosphere that consists of: 21% oxygen, 0.04% carbon dioxide, 78% nitrogen gas and a varying amount of water vapor (1-4%) and certain noble gases, etc.
Water vapor and carbon dioxide have the ability to absorb heat (long-wave radiation) and later send it away again. This means that the atmosphere retains a lot of heat near the earth’s surface – where the atmosphere is wetter and has the highest pressure.
Human additions of carbon dioxide to the atmosphere are small when we compare with a real volcanic eruption. Of the atmosphere’s 0.04% CO2, humans are said to contribute with 3.4%, ie. 3.4% of 0.04% gives: 0.00136%, 13.6 ppm.
Here comes a laboration that demonstrates the temperature-reducing effect of carbon dioxide of the atmosphere. The lab can be done in classrooms or at home.
Laboratory experiment arrangement
Task: Determine if carbon dioxide has a warming or cooling effect as “greenhouse gas”.
Material: 2 thermometers (preferably digital thermometers), two mineral water bottles with carbonated water, aluminum foil, silver tape, space behind sunny window.
Arrangement: The thermometers must be synchronous, i.e. showing the same “temperature” (oC) at the same temperature. They should show 0 oC in ice water and 100 oC in boiling water. If they would deviate a little, then one must find out how they differ from one another.
Remove the labels from the pet bottles. It’s easy if the labels are plastic and are loose. Pour water from the pet bottles so that there is about 7 cm of water left in one bottle. Let all the carbonic acid (carbon dioxide solved in water) in this bottle be degassed for a few days. Shake the bottle and have open cap. Make a small hole in the cap so that either a laboratory thermometer (rod thermometer) or digital thermometer can have its sensor about 3 cm down in the bottle. You can melt hole in the cap with a metal awl heated over a candle. Fix the thermometer’s sensor with duct tape and don’t leave the sensor in contact with the inner wall of the bottle but it should be free. Make sure the cap of the second bottle also gets a hole with the thermometer sensor in the same way as in the first bottle. Fix with duct tape and seal as good as you can. Let the amount of water in the second bottle be equal to that in the first bottle. Leave the carbonic acid in the bottle. Cover the top of the pet bottles equally with aluminum foil so that the sun cannot shine directly on the sensors. Leave both bottles behind window towards the south and wait for sunshine. Make sure the gas pressures in the bottles are the same, i.e. gas must be able to pass out or into the bottles depending on different air pressures – there must be the same pressure conditions in both bottles. This is achieved even if you seal well with duct tape.
Set-up: acc. figure below, and as described in the text under “arrangement”. You can execute the experiment in different sunny windows to see possible deviations.
Result: The consistency of the thermometers: The thermometers show the same temperature at the same “temperature”.
The picture shows the experiment at night at room temperature (the radiator is not on).
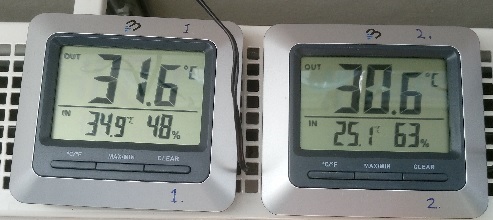
Sunlight temperature (pictured below): bottle # 1, which has only atmospheric water vapor above the surface of the water (common air), starts to get a higher temperature compared to bottle # 2, which has extra carbon dioxide in its atmospheric gas. As can be seen in the picture below, the temperatures differ about one degree Celsius.
At night without sun exposure, it was the same temperature in both bottles. So it was night after night and even day after day when the sun was not on. The temperature was the same in both bottles. As soon as the sun began to shine on the experiment and the temperature increased, a temperature deviation between the bottles of about a degree Celsius occurred. The experiment was up for about two weeks and the result was the same every time the sun shone on the bottles and especially when the temperature reached 30 degrees or more. Initially, the carbon dioxide concentration in bottle 2 needed to decrease a little before we reached the elevated carbon dioxide levels that caused the temperature drop during sunshine.
Conclusion: Carbon dioxide (in this experiment) seems to reduces the heat-holding effect of the atmosphere, and contributes to cooling of the atmosphere, compared to water vapor. Carbon dioxide contributes to a decreasing “greenhouse effect”, and has a cooling effect on the Earth’s atmospheric climate, as greenhouse gas.
The picture above shows the sun shining on the pet bottles. Below is the temperature in the two bottles.
Discussion:
The ideal gas law:
p * V = n * R * T
p = pressure (N / m2)
V = volume (m3)
n = amount of substance (number of moles; 1 mole = 6.02 * 1023 pcs of molecules or atoms)
R = gas constant (8.3145 J / (mol * K))
T = absolute temperature (K (Kelvin))
In the experiment, p, V, R can be considered constant. This means that as the temperature rises, the number of molecules in the gas decreases. Similarly, as the temperature decreases, more molecules are allowed into the atmosphere of the bottle.
As the temperature rises, collisions of gas particles increase. The pressure is the same in both bottles, which means that there are fewer gas particles in bottle 1 (air) compared to bottle 2 (air and extra carbon dioxide). As bottle 2 has a lower temperature, more gas particles are allowed to be present in the atmosphere of the bottle.
An addition of carbon dioxide to the air causes some water vapor to condense into water. This can be seen if two pet bottles like bottle # 1 are set up (regular air in both). Let these bottles be for a while. Then in bottle 2 dissolve a little baking soda in the water and a little acetic acid is then poured in. The chemical reaction that takes place creates carbon dioxide which goes up in the atmosphere of the bottle according to the reaction:
Sodium hydrogen carbonate (baking soda) + acetic acid –> sodium acetate + water + carbon dioxide
NaHCO3 + CH3COOH –> CH3COONa + H2O + CO2
This makes water vapor condense on the inside of the bottle (or disappear from the bottle). The heat-retaining property of the atmosphere (“greenhouse effect”) decreases and the temperature drops. Carbon dioxide is a poorer greenhouse gas compared to water vapor, which means that an extra carbon dioxide addition to the earth’s atmosphere lowers its temperature. Thus, it cannot be said that carbon dioxide would raise the temperature of the earth’s atmosphere, since water vapor is not allowed in the atmosphere in the same amount as before the carbon dioxide addition.
The result is that Carbon Dioxide reduces the Earth’s atmospheric current greenhouse effect and temperature.
Here are sources of error for this lab which must be taken into account. This laboratory does not show an absolute scenario for the greenhouse effect for the set up leaves some question marks and the interactions between the incoming gases are also complicated as they all have different properties.
That the cork is open with a little leakage:
==================================
When the sun is on, gas from the bottle will leave the bottle. To say with this experiment that both bottles leak the same amount of gas – that would be a false statement. As the sun diminishes, outside air enters the bottle – a small “breath” of the bottle. On the other hand, under constant sunshine and rising temperatures, we can say that the flow of gas should be directed outward from the bottles.
The temperature sensor:
==================================
This should be centered in the bottle and at the same height. Nor can this be guaranteed.
Convection:
==================================
Bottle No. 2 with carbon dioxide may have two main convection cells (warmer air rises and colder drops). Carbon dioxide is a heavier (CO2; 0.04%, 44 g/mol) gas than air containing mainly Nitrogen gas (N2, 78%, 28 g/mol), Oxygen (O2, 21%, 32 g/mol) and Argon (Ar, 1%, 40 g/mol). We assume that if the carbon dioxide content is quite high in the experiment, then carbon dioxide itself will have convection cells above the water surface. Remained air will have convection cells above these, and could also have a lower temperature. Now this experiment has also been done with the temperature sensors placed further down in the bottles as well, and the experiment has still shown that when the sun is on, bottle # 2 with a little added carbon dioxide has a lower temperature. We assume that the carbon dioxide is mixed in the experiment when its content is low and the lid is minimal-leakage-open. To minimize low-temperature convection above the carbon dioxide convection cells, the aluminium foil would stop this, whereas the sun cannot shine through the aluminium foil and heat the gas in the upper part of the bottle where the temperature sensor is.
Warning – there are errors in laboratory set ups that want to show that carbon dioxide raises atmospheric temperature. – These are carried out today in schools.
==================================
Several laboratory set-ups with a reference bottle have been set up in schools around the world to demonstrate the warming effect of carbon dioxide in an atmosphere. These experiments have been designed to show that carbon dioxide is responsible for the temperature rise between the years 1980 and 2000. Experiments have been set up to “prove” this. Here are the two most common laboratory setups that show this fraud.
IR lamp on reference pet bottle with air (1) and bottle with carbon dioxide (2):
==================================
No water is in the bottles. There can be air in one bottle (1) and only carbon dioxide in bottle 2. The bottle with air then contains: 21% oxygen, 78% nitrogen, 1% Argon and minimal water vapor and carbon dioxide. The second bottle then contains pure carbon dioxide gas (100%). It goes without saying that the second bottle with carbon dioxide alone will show a higher temperature, since carbon dioxide is a greenhouse gas. The first bottle contains transparent gases (oxygen, nitrogen) that do not absorb solar radiation. In this way, one cannot set up an experiment as it goes without saying that a bottle with only greenhouse gas shows the highest temperature. With such an arrangement you want to show that a higher carbon dioxide atmosphere shows higher temperature.
E.g. these types of laboratory experiments:
https://www.rsc.org/Education/Teachers/Resources/jesei/co2green/home.htm
https://serc.carleton.edu/eslabs/weather/2d.html
In the reference bottle with ordinary air, the humidity will not increase significantly – which is normal in nature. More water vapor will not be supplied to the air, which means that hardly any extra water vapor can come to the rescue and help store the heat in the bottle’s atmosphere.
IR lamp
==================================
It is not possible to use an IR lamp because the IR lamp (short and long-wave heat radiation) is not the same as sunlight. The IR lamp heats the CO2 gas directly (not “greenhouse effect”) but cannot heat the air in the reference bottle. The sun is very far away.
Absence of water in the bottles
==================================
70% of the earth is covered with water and water is found in soil and atmosphere. As the carbon dioxide content increases, water will condense. A good greenhouse gas (water) will be replaced by a bad greenhouse gas (carbon dioxide). The heat-retaining effect in the atmosphere gets worse and the “system” works to counteract a rise in temperature. In several school experiments, water is missing in the containers and the phase transitions of water are not taken into account.
Specific heat capacity
==================================
To raise air 1 degree centigrade or Kelvin, 1.01 KJ / (kg * K) is needed. Here comes specific heat capacity for some gases.
Air 1.01 KJ / (kg * K)
CO2 0.84 KJ / (kg * K)
Ar 0.52 KJ / (kg * K)
Cl2 0.48 KJ / (kg * K)
CH4 2.22 KJ / (kg * K)
H2O 4,19 KJ / (kg * K)
Thus, less energy is needed to heat carbon dioxide one degree compared to air. From this, the temperature rises faster in carbon dioxide. The temperature rises even faster in chlorine gas.
Baking powder and acetic acid
==================================
When using baking powder and acetic acid in water to produce carbon dioxide and using an excess of acetic acid. Here you can feel the smell in the bottle that its atmosphere is stinging. Acetic acid dissolves into the water vapor and we get dissolved acetic acid in the water vapor. In order for this experiment to be reasonably in accordance with nature, it must be calculated exactly how much acid is needed and exactly how much baking powder is needed to raise the carbon dioxide content to a certain level, e.g. 900 ppm or 1% or similar. Everything must react in the reaction and no extra acetic acid may be present, which dissolves in the water vapor and gives a pungent odor. This experiment Adrian Vance has done and reported in his book “Vapor Tiger”. He shows that at 910 ppm carbon dioxide, the atmospheric temperature drops 1 degree compared to normal air.
Extra applicable dimensions of the universe:
==================================
This experiment is three-dimensional with the room’s three axes x, y, z and time. The universe is made up of more dimensions (10 in total) which also obviously affect the experiment, but much research remains.
Bottle Material
==================================
This material can absorb some type of radiation – here may be a source of error.
Using tap water
==================================
Tap water may contain e.g. chlorine gas or other additives that could affect the result. Chlorine gas has a lower specific heat capacity and could give a temperature increase. Use mineral water instead.
Other
==================================
We will update this lab further. If you have any comments, please enter them below in the comments field or send an email.
References
==================================
https://principia-scientific.org/the-deliberately-false-greenhouse-gas-co2-experiment/
http://www.climatechangeeducation.org/hands-on/difficulties/heating_greenhouse_gases/
http://www.rsc.org/Education/Teachers/Resources/jesei/co2green/home.htm
https://serc.carleton.edu/eslabs/weather/2d.html
https://www.researchgate.net/figure/Greenhouse-Gas-Bottle-Demonstration-97-Bottles-with-CO2-and-air-are-radiated-and_fig77_324751934
http://chem-www4.ad.umu.se:8081/Skolkemi/Experiment/experiment.jsp?id=74
The book “Vapor Tiger” by Adrian Vance (recommended reading).
https://webapps.kemi.se/flodesanalyser/Amnesinformation/attiksyra_sv.htm
After publication…translate to English…
24/6-2021: The sun without greenhouse effect (Solen utan ‘växthuseffekt’):
https://klimatsans.com/2021/06/24/solen-utan-vaxthuseffekt/
7/7-2021: Earth’s ‘energy budget’ – a physical falsarium (Jordens ‘energibudget’-ett fysikaliskt falsarium):
https://klimatsans.com/2021/07/07/jordens-energibudget-ett-fysikaliskt-falsarium/
14/7-2021: Carbon dioxide: Cooling Only (Koldioxid: Endast kylande):
https://klimatsans.com/2021/07/14/koldioxid-endast-kylande/
The search engine google shows in its search results only bottle labs which, on closer examination, mislead to give a definite view that extra carbon dioxide to the atmosphere raises the atmospheric temperature. In the experiment above, we show the opposite. Use the search engine e.g. Yahoo instead to compare.
Speculatively, a political agenda is now being conducted that says that the slight increase in temperature that occurred between 1980 and 2000 would be due to an increase in carbon dioxide concentration in the atmosphere. Temperature data has been manipulated and they want to get the carbon dioxide content in the atmosphere to exactly follow a manipulated temperature curve from the beginning of the 20th century, to show a connection here. It should be mentioned that carbon dioxide has only 1/7 of the water-holding property of the water molecule and that the water molecules can be up to 150 times more in the atmosphere. Carbon dioxide is of minor importance and cannot be said to significantly affect the temperature of the earth’s atmosphere. As carbon dioxide gas is released into the atmosphere, water vapor will condense. This causes the temperature to drop when a good greenhouse gas (water vapor) is replaced by a poor “greenhouse gas” (carbon dioxide).
 Unifier, World Teacher News! A new consciousness in a new time. The sole solution of all world problems. Achieve a higher quality multi-dimensional quantum consciousness. There are no other ways around.
Unifier, World Teacher News! A new consciousness in a new time. The sole solution of all world problems. Achieve a higher quality multi-dimensional quantum consciousness. There are no other ways around.